Isolation, Characterization, Antimicrobial and Theoretical Investigation of Some Bioactive Compounds Obtained from the Bulbs of Calotropisprocera
Keywords:
Phytochemical Screening, Anti-microbial screening, Calotropis procera, PharmacokineticsAbstract
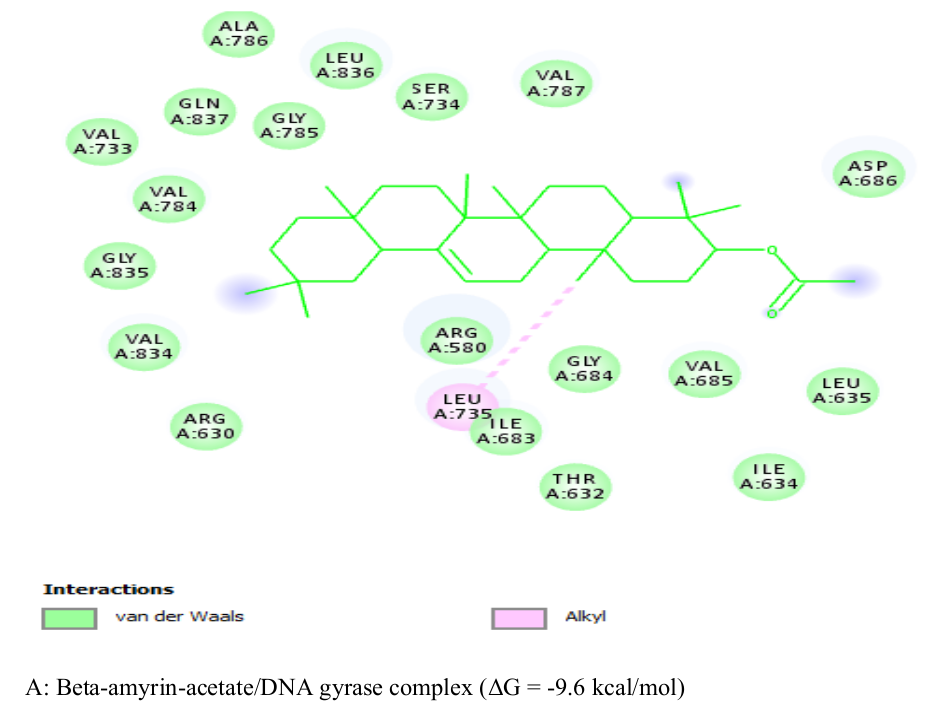
This study characterizes the bioactive molecules from the bulb of Calotropisprocera and investigates the antimicrobial activities of the crude extracts. Theoretical studies on the two isolated compounds in the crude extract were also accomplished.The bulbs were air dried, pulverized, and subjected to extraction procedures by maceration using 500 mL each of normal-hexane, ethyl acetate and methanol. The crude extracts were further tested onmicroorganisms and phytochemical screening using standard procedures. In addition, the bioactive compounds in the extract were screened against DNA gyrase of two Gram negative bacterial species; Escherichia coli and Salmonella typhiusing Molecular Docking simulation techniques and further subjected to ADMET profiling,using the Swiss ADME online server. The Crude ethyl acetate extract has the highest effective activity against Escherichia coli (MIC 2.5mg / mL and MBC/MFC 5mg / mL), Staphylococcus aureus (MIC 2.5mg/mL), Candida albicans, Salmonella typhiand Candida stellafoidea (MIC 5mg/mL). beta-Amyrin acetate and Taraxasterol are the two phytochemicals in the purified white crystalline fractions and were found to fasten to the active sites of DNA gyrase of the Gram negative bacterial species via hydrophobic and hydrogen bond interactions, with binding activity value of -9.6 kcal/mol and -9.5 kcal/mol, respectively. Also, ADMET investigations of the compounds revealed their sound oral bioavailability and excellent pharmacokinetic and toxicity profiles. The findings of this study could provide a platform for discovering safe and potent antibiotics against pathogenic microbes ravaging our society.
Published
How to Cite
Issue
Section
Copyright (c) 2023 M. E. Khan, C. E. Elum, A. O. Ijeomah, P. J. Ameji, I. G. Osigbemhe, E. E. Etim, J. V. Anyam, A. Abel, C. T. Agber

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- Bethel Onyeka Ekute, Muluh Emmanuel Khan, Aloysius Akaangee Pam, Jude Ehwevwerhere Emurotu, Assessment of the nutritional and phytochemical composition of selected mushroom species grown in Southern Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 4, November 2025
- Kazeem A. Tijani, Chinwendu E. Madubueze, Reuben I. Gweryina, Typhoid fever dynamical model with cost-effective optimalcontrol , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 4, November 2023
- Chidi Duru, Ijeoma Duru, Chiagoziem Chidiebere, Virtual Screening of Selected Natural Products as Human Tyrosinase-Related Protein 1 Blockers , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 3, August 2021
- E. P. Onokare, L. O. Odokuma, F. D. Sikoki, B. M. Nziwu, P. O. Iniaghe, J. C. Ossai, Physicochemical Characteristics and Toxicity Studies of Crude Oil, Dispersant and Crude Oil-Dispersant Test Media to Marine Organisms , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 1, February 2022
- John Tulirinya, Mathew Kinyanju, Samuel Mutua, Asaph Muhumuza, Optimizing initial chlorine dosage at an injection point along a water distribution pipe , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 4, November 2025
- B. Bako, E. E. Etim, J. P. Shinggu, S. S. Humphrey, L. J. Moses, M. E. Khan, Quantum chemical calculations of lupeol (C30H50O) isolated from the ethyl acetate leaf extracts of Justicia Secunda , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 3, August 2024
- B. T. Iorhuna, T. T. Awuhe, I. C. Azuaga, E Isaac, F. Shuaibu, B. Yohanna, Synthesis, Characterization and Antimicrobial Activities of Copper-Tea Leaves (Camellia Sinensis) Extract Nanoparticles.: None , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 4, November 2022
- S. D. Umoh, A. K. Asekunowo, I. S. Okoro, N. X. Siwe, R. W. M. Kraus, O. O. Okoh, A. O. T. Ashafa, O. T. Asekun, O. B. Familoni, Antioxidant evaluation and bio-guided isolation from methanol leaf extract of Acalypha godseffiana , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 3, August 2024
- C. A. Onate, I. B. Okon, J. A. Akinpelu, O. O. Ajani, O. A. Adedewe, B. B. Deji-Jinadu, Shannon entropy and thermodynamic properties of an inversely quadratic Yukawa potential model , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 4, November 2024
- C. E. Duru, C. E. Enyoh, I. A. Duru, M. C. Enedoh, Degradation of PET Nanoplastic Oligomers at the Novel PHL7 Target:Insights from Molecular Docking and Machine Learning , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 1, February 2023
You may also start an advanced similarity search for this article.
Most read articles by the same author(s)
- E. E. Etim, Benchmark Studies on the Isomerization Enthalpies for Interstellar Molecular Species , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 2, May 2023






