Quantum chemical calculations of lupeol (C30H50O) isolated from the ethyl acetate leaf extracts of Justicia Secunda
Keywords:
Justicia secunda, Lupeol, Quantum chemical CalculationsAbstract
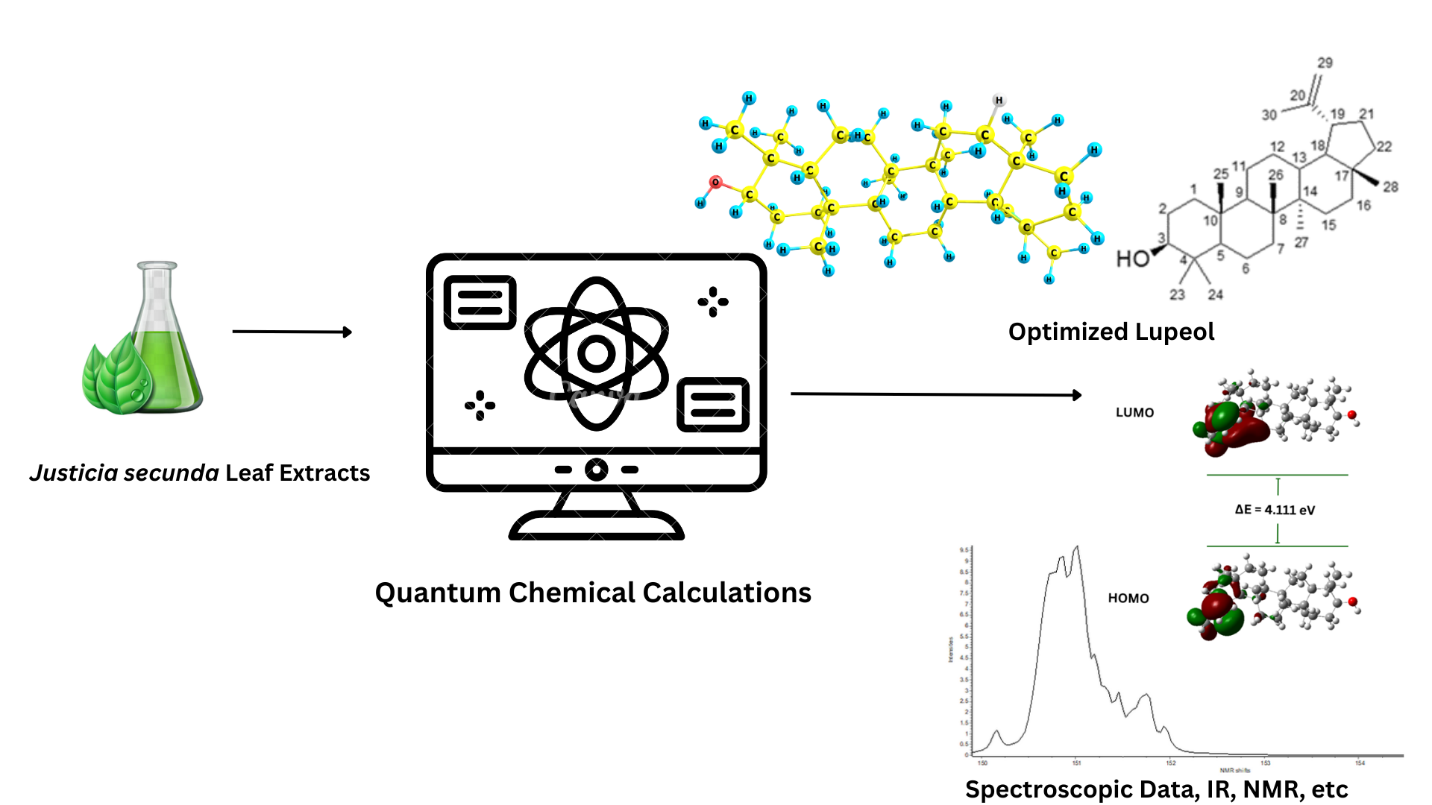
The discovery of lupeol, a triterpenoid compound (C30H50O), in the ethyl acetate leaf extract of Justicia secunda (Blood root), has opened doors to extensive research and development opportunities in natural product-based pharmaceuticals. Lupeol’s versatile pharmacological properties, including anti-inflammatory, anticancer, antidiabetic, and antiviral effects, make it a compelling candidate for drug development. To fully harness its potential, a comprehensive understanding of lupeol’s structural and chemical attributes is crucial. Through quantum chemical calculations using the GAUSSIAN 09 suite of programs, we determined the optimized geometry, IR frequencies, bond distances (R), bond angles (A), dipole moments, HOMO-LUMO and other molecular parameters for this solitary molecule. The remarkable accuracy and reliability of computational techniques in predicting the properties of systems and reactants are evident in the consistently favorable results. A strong concordance and consistency between the experimental and computational outcomes further reinforces the credibility of our findings. This study offers a means to explore lupeol’s molecular behavior, providing insights that can guide future drug development efforts rooted in this promising natural compound.
Published
How to Cite
Issue
Section
Copyright (c) 2024 E. E. Etim, B. Bako, J. P. Shinggu, S. S. Humphrey, L. J. Moses, M. E. Khan

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- M. E Khan, E. E. Etim, V. J Anyam, A Abel, I. G Osigbemhe, C. T Agber, Computational studies on Emodin (C15H10O5) from Methanol extract of Pteridium acquilinum leaves , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- E. E Etim, M. E Khan, O. E Godwin, G. O Ogofotha, Quantum Chemical Studies on C4H4N2 Isomeric Molecular Species , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- B. T. Ogunyemi, F. K. Ojo, Corrosion Inhibition Potential of Thiosemicarbazide Derivatives on ALuminium: Insight from Molecular Modelling and QSARs Approaches , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 1, February 2023
- C. W. Chidiebere, C. E. Duru, JP. C. Mbagwu, Application of computational chemistry in chemical reactivity: a review , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- C. B. Adindu, S. C. Nwanonenyi, C. B. C. Ikpa, Experimental and computational studies of the corrosion inhibitive effects of Zingiber officinale rhizomes on mild steel corrosion in acidic solutions , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 3, August 2023
- W. A. Yahya, A. A. Yahaya, A. A. Adewale, A. A. Sholagberu, N. K. Olasunkanmi, A DFT study of optoelectronic, elastic and thermo-electric properties of the double perovskites Rb2SeX6 (X=Br,Cl) , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 2, May 2023
- Muteeu A. Olopade, Anthony B. Adegboyega, Kayode I. Ogungbemi, Adeyinka D. Adewoyin, Investigation of the behaviour of tunable chalcogenide-Bismuth based perovskite BiTl (SxSe1-x)3(X = 0, 0.33, 0.67, 1): first principles calculations , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 1, February 2025
- F. L. Kherfane, A. BOUKRAA, B. Beladel, A. Douara, I. E. Tibermacine, A. Rabehi, M. Benghanem, Structural, electronic and optical properties of theFeAs(1-x)La(x) ternary alloys: a first principles calculations , Journal of the Nigerian Society of Physical Sciences: Volume 8, Issue 1, February 2026 (In Progress)
- Saprizal Hadisaputra, Lalu Rudyat Telly Savalas, Corrosion Inhibition Properties of Lawsone Derivatives againts Mild Steel: A Theoretical Study , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 3, August 2023
- A. Shamsudeen, Shuaibu S., S.G. Abdu, M. S. Abubakar, Abdullahi lawal, First-principles calculations of Fluorine-doped Titanium dioxide: A prospective material for solar cells application , Journal of the Nigerian Society of Physical Sciences: Volume 1, Issue 4, November 2019
You may also start an advanced similarity search for this article.
Most read articles by the same author(s)
- M. E Khan, E. E. Etim, V. J Anyam, A Abel, I. G Osigbemhe, C. T Agber, Computational studies on Emodin (C15H10O5) from Methanol extract of Pteridium acquilinum leaves , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021






