Computational investigation and biological activity of selected Schiff bases
Keywords:
Schiff base, DFT, Molecular docking, AntibacterialAbstract
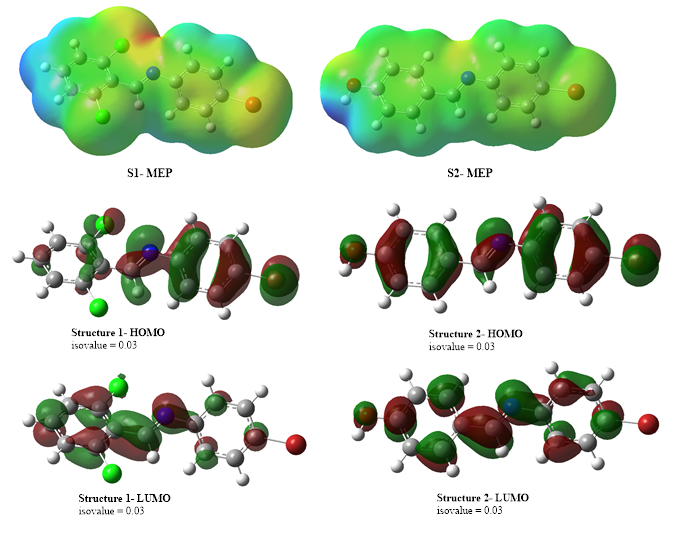
Two Schiff bases, (E)-N-(4-bromophenyl)-1-(2,6-dichlorophenyl)methanimine (S1) and (E)-4-(((4-bromophenyl)imino)methyl)phenol (S2) were synthesized and elucidated using basic spectroscopic methods such as Fourier-transform-infrared, Ultraviolet-visible as well as 1H & 13C-NMR while purity of the compounds was ascertained by the means of elemental analysis. The stability, chemical reactivities and electronic properties of S1 and S2 were evaluated using Density Functional Theory (DFT). Energy band gap (?E) of S1 and S2 are 2.81 eV and 3.02 eV respectively and this inferred that S1 is more reactive as well as less stable than S2. The Molecular electrostatic potential (MEP) mapping evaluation showed that both S1 and S2 are having more of the yellow, green and red region than the blue region, signifying that these structures are prone to electrophilic attack. The compounds were screened against one Gram-negative (Staphylococcus aureus) and two Gram-positive bacteria (Klebsiella pneumoniae and Psedomonas aeruginosa) to evaluate their antibacterial potential. S1 as well as S2 showed antibacterial potential against Staphylococcus aureus and exhibited less of no activity against Klebsiella pneumoniae and Escherichia coli. In silico studies of S1 and S2 against Staphylococcus aureus was carried out and the outcomes corroborate with experimental findings. Pharmacological evaluation of S1 and S2 showed that both compounds exhibited less violation of Lipinski’s rule of five (Ro5) which pose them to be less toxic and orally available.
Published
How to Cite
Issue
Section
Copyright (c) 2024 Segun Oladipo, Adesola A. Adeleke, Abosede A. Badeji, Katherine I. Babalola, Ayomide H. Labulo, Ibrahim Hassan, Sadiq T. Yussuf, Samuel O. Olalekan

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- Nassima Bou-ydia, Ben-issa El miraoui, Latifa Laallam, Ahmed Jouaiti, Interaction of hydroxyapatite and chitosan with gentamicin and their antimicrobial activities: DFT and molecular docking approach , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 3, August 2025
- I. E. Otuokere, J. G. Ohwimu, K. C. Amadi, C. O. Alisa, F. C. Nwadire, O. U. Igwe, A. A. Okoyeagu, C. M. Ngwu, Synthesis, Characterization and Molecular Docking Studies of Mn (II) Complex of Sulfathiazole , Journal of the Nigerian Society of Physical Sciences: Volume 1, Issue 3, August 2019
- K. O. Eberendu , J. I. Iheanyichukwu, O. M. Mac-kalunta, C. I. Nwankwo, I. E. Otuokere, J. C. Nnaji, Zn(II) and Fe(II) complexes of 2,4-dinitro-N-[(Z)-[(E)-3-(2-nitrophenyl)prop-2-enylidene] amino] aniline: synthesis, characterization and In Silico SARS-CoV-2 inhibition studies , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 1, February 2025
- B. T. Ogunyemi, F. K. Ojo, Corrosion Inhibition Potential of Thiosemicarbazide Derivatives on ALuminium: Insight from Molecular Modelling and QSARs Approaches , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 1, February 2023
- Misbaudeen Abdul-Hammed, Ibrahim Olaide Adedotun, Tolulope Irapada Afolabi Afolabi, Ubeydat Temitope Ismail, Praise Toluwalase Akande, Balqees Funmilayo Issa, Analysis of the Bioactive Compounds from Carica papaya in the Management of Psoriasis using Computational Techniques , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 1, February 2023
- U. B. Amadi, M. Ogwuegbu, C. K. Enenebeaku, G. onyedika, Design, synthesis and studies on thermodynamic and biological activities of lanthanide III complexes of hydrazinecarbothioamide ligands , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 2, May 2025
- C. E. Duru, C. E. Enyoh, I. A. Duru, M. C. Enedoh, Degradation of PET Nanoplastic Oligomers at the Novel PHL7 Target:Insights from Molecular Docking and Machine Learning , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 1, February 2023
- M. E. Khan, C. E. Elum, A. O. Ijeomah, P. J. Ameji, I. G. Osigbemhe, E. E. Etim, J. V. Anyam, A. Abel, C. T. Agber, Isolation, Characterization, Antimicrobial and Theoretical Investigation of Some Bioactive Compounds Obtained from the Bulbs of Calotropisprocera , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 3, August 2023
- A. H. Labulo, A. D. Terna, O. F. Oladayo, H. Ibrahim, N. S. Tanko, R. A. Ashonibare, J. D. Opeyemi, Z. Tywabi-Ngeva, Photocatalytic and antibacterial activities of green-mediated Khaya senegalensis-silver nanoparticles and oxidized carbon nanotubes , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 3, August 2023
- Ogheneovo Akpoyibo, Ezekiel Onoriode Abriku, F. C. Ugbe, Ochuko Anomohanran, Geophysical and geotechnical assessment of Obiaruku-Agbor road failure in Western Niger-Delta, Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 1, February 2025
You may also start an advanced similarity search for this article.






