Synthesis and in vitro bioactivity of sodium metasilicate-derived silicon-substituted hydroxyapatite
Keywords:
Silicon-substituted hydroxyapatite, Bioactivity, Sodium metasilicate, Bone repair, Synthetic bone graftsAbstract
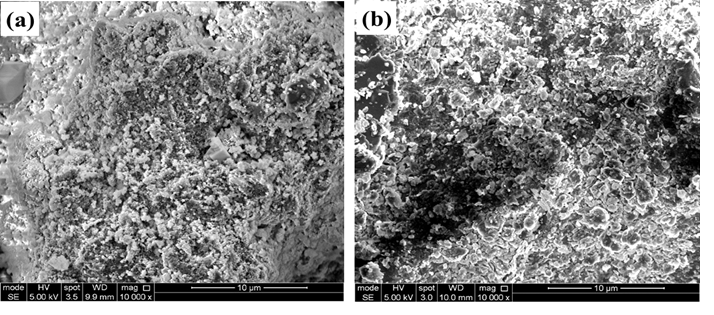
Structural alteration of synthetic implants aims to achieve better bioactivity, higher cellular response, and regulated degradability, all of which are critical criteria for a biomaterial to serve as a graft in bone regeneration. The aim of this work was to synthesize silicon-substituted hydroxyapatite and test its bioactivity in simulated body fluid (SBF) by proving the use of sodium metasilicate (Na2SiO3.9H2O) as an affordable precursor of silica. Thus, the study evaluated the in vitro bone-bonding capacity of hydroxyapatite (Ca10(PO4)6(OH)2) (HA) substituted with silicate ion (Ca10(PO4)6-x(SiO4)x(OH)2-x; SixHA). The SixHA with x = 0.4 was synthesized by utilizing a wet precipitation method with sodium metasilicate as a low-cost silica alternative for alkoxysilane precursors. The SixHA was then examined for properties such as morphology, elemental composition, phase composition, and the nature of chemical bonds using scanning electron microscopy (SEM), energy dispersive X-ray analysis (EDX), X-ray diffractometry (XRD), and Fourier transformed infrared spectroscopy (FTIR), respectively. An in vitro bioactivity experiment was also carried out by incubating the SixHA in simulated body fluid (SBF) at 36.5 °C for 7 and 14 days. The obtained results revealed the substitution of SiO44- for some PO43- groups in the hydroxyapatite structure. The SixHA nucleated more apatite crystals on its surface and demonstrated some degradability during the periods of immersion in SBF. The characteristics of the SixHA imply that it could be used as a graft in bone restoration applications, thus signifying that sodium metasilicate could serve as an economic silica source for silicon-substituted hydroxyapatite production.
Published
How to Cite
Issue
Section
Copyright (c) 2024 Enobong R. Essien, Violette N. Atasie, Ngozi A. Adeleye, Luqman A. Adams

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- K. M. Omatola, A. D. Onojah, R. Larayetan, A. O. Ohiani, I. I. Oshatuyi, M. B. Ochang, O. Anawo, P. Abraham, Isolation and investigation of the structure of silicon quantum dots from rice husk ultrafine silica for possible applications in nanoelectromechanical systems , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 4, November 2025
- K. M. Omatolaa, A. D. Onojah, A. N. Amah, I. Ahemen, Synthesis and characterization of silica xerogel and aerogel from rice husk ash and pulverized beach sand via sol-gel route , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 4, November 2023
- Nassima Bou-ydia, Ben-issa El miraoui, Latifa Laallam, Ahmed Jouaiti, Interaction of hydroxyapatite and chitosan with gentamicin and their antimicrobial activities: DFT and molecular docking approach , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 3, August 2025
- A. A. Faremi, S. S. Oluyamo, K. D. Adedayo, Y. A. Odusote, O. I. Olusola, Influence of Silicon Nanoparticle on the Electrical Properties of Heterostructured CdTe/CdS thin films based Photovoltaic Device , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 3, August 2021
- Anas Sani Maihulla, Ibrahim Yusuf, Performance Analysis of Photovoltaic Systems Using (RAMD) Analysis , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 3, August 2021
- Raphael Ozighor Enihe, Rajesh Prasad, Francisca Nonyelum Ogwueleka, Fatimah Binta Abdullahi, The effect of imbalance data mitigation techniques on cardiovascular disease prediction , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 2, May 2025
- A. M. Olaosun, C. A. Aborisade, D. E. Shian, P. T. Osuolale, Comprehensive study of photon and proton interactions to interpret the radiation parameters of boron derivative drugs for chemoradiotherapy , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 2, May 2025
- L. G. Salaudeen, D. GABI, M. Garba, H. U. Suru, Deep convolutional neural network based synthetic minority over sampling technique: a forfending model for fraudulent credit card transactions in financial institution , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 2, May 2024
- Elsayed Elshoubary, Effect of reduction method on the performance a software defined network system using Gumbel Hougaard family copula distribution , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 4, November 2023
- Chidi Duru, Ijeoma Duru, Chiagoziem Chidiebere, Virtual Screening of Selected Natural Products as Human Tyrosinase-Related Protein 1 Blockers , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 3, August 2021
You may also start an advanced similarity search for this article.






