Modeling the dynamics of pertussis to assess the influence of timely awareness with optimal control analysis
Keywords:
Dynamics, Optimal control, Mathematical model, Control reproduction number, PertussisAbstract
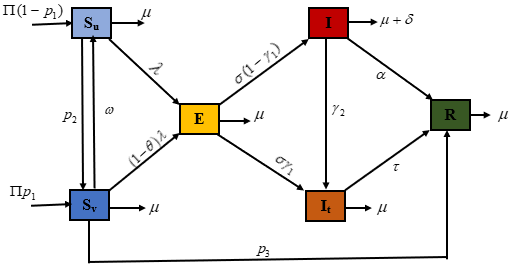
Pertussis, also known as whooping cough, is a very infectious respiratory disease that can be easily avoided with vaccination. For newborns, whooping cough poses an especially serious risk. In addition to a ”whoop”-like cough, other symptoms include sneezing, nasal congestion, and a runny nose. The bacteria that cause pertussis are called Bordetella pertussis. The upper respiratory system is the main target of the disease, and it is extremely contagious. In this work, a system of nonlinear ordinary differential equations of pertussis has been formulated to examine the impact of early treatment. Through theoretical examination, the positivity and boundlessness of the solution are confirmed. Furthermore, the local stability has been examined using the Jacobian matrix, and the equilibrium points for the system are derived for both the free and the endemic instances, and global asymptotic stability of disease-free equilibrium have been ascertained using the comparison method, which shows that the disease-free equilibrium is globally asymptotically stable if the control reproduction number is less than one. Furthermore, the global asymptotic stability of the endemic equilibrium point was determined using the Lyapunov function of the Goh-Volterra type, which shows that the endemic equilibrium point is globally asymptotically stable if the control reproduction number is greater than one. Numerical experiments are performed to validate the theoretical conclusions. The suggested model has been fitted to real Austrian pertussis data, demonstrating that it is appropriate for the data. The control reproduction number was also used to test the sensitivity analysis of all of the parameters of the proposed model. The results indicate that the effective contact rate is the parameter that is more sensitive to increasing the control reproduction number. In contrast, the awareness rate is the parameter that is most sensitive to decreasing the number of control reproductions, and optimal control analysis has also been performed in this work. Numerical simulation reveals that awareness is the most influential parameter in reducing infection in the population. Moreover, vaccination and treatment are also very important in controlling pertussis in society.
Published
How to Cite
Issue
Section
Copyright (c) 2025 J. Andrawus, J. Y. Musa, S. Babuba, A. Yusuf, S. Qureshi, U. T. Mustapha, A. Oghenefejiro, I. S. Mamba (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.







