Eigensolutions and thermodynamic properties constrained by magnetic field of cyclotron frequency
Keywords:
Bound state, Eigensolution, Thermal properties, Partition function, Potential modelAbstract
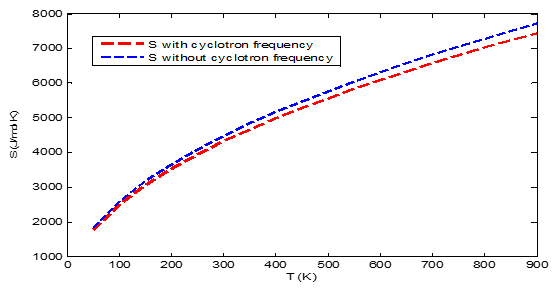
The present work investigates the influence of cyclotron frequency on the energy spectrum and thermodynamic properties of a quantum system. The energy as a function of the quantum number unveils an increase in the system's energy due to the presence of cyclotron frequency, making it open-ended by curtailing its negativity. The screening parameter significantly shapes the system’s energy, increasing at the initial point due to Landau quantization and then monotonically decreasing as a result of weakness of Coulomb interaction. The study also revealed that the parameter a and b impacted the energy differently as the cyclotron frequency altering the system’s confinement behaviours. Similarly, the partition function (Z) and some thermodynamic properties (TP), are analyzed under varying temperatures and parameter conditions. The presence of the cyclotron frequency (CF) reduces the Z and the TP due to Zeeman effect. The TP (H and S) exhibited similar characteristics. Thus, the presence of CF affects the stability of the system. This study finds applications in quantum wells and dots, fusion energy, magnetic quantum computing, and quantum statistical mechanics.
Published
How to Cite
Issue
Section
Copyright (c) 2025 C. A. Onate, S. O. Mobolaji, J. A. Akinpelu (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.







