Assessment of the nutritional and phytochemical composition of selected mushroom species grown in Southern Nigeria
Keywords:
Proximate analysis, GC-MS analysis, Macro-minerals, TerpenoidsAbstract
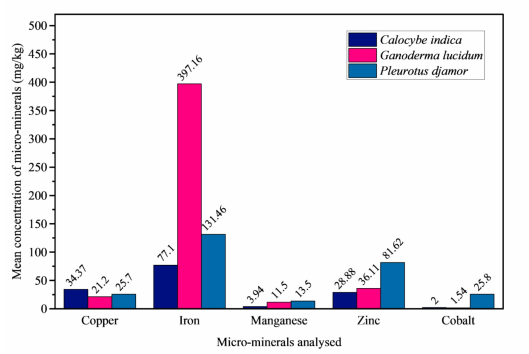
Mushrooms are increasingly gaining attention for their nutritional and therapeutic benefits due to their rich composition of essential nutrients and bioactive compounds. However, all-encompassing integrative data on Calocybe indica, Ganoderma lucidum, and Pleurotus djamor grown in southern Nigeria is lacking. This study aims to provide a detailed, parallel evaluation of the nutritional and phytochemical composition of these mushroom species through a multidimensional analysis. The proximate, mineral, and phytochemical contents of the mushrooms were determined following standard analytical methods, while the metabolites were identified using gas chromatography-mass spectrometry (GC-MS). The phytochemical, terpenoid, was of the highest level (2.25 ± 0.05 % to 15.91 ± 0.41 %), with Ganoderma lucidum having the highest value. In the GC-MS chromatograms of the methanol extracts of the mushrooms, the most prominent bioactive metabolites were cis–vaccenic acid (31.32 %) and n-hexadecenoic acid (27.75 %), ergosterol (28.02 %), and linoelaidic acid (37.83 %) for Calocybe indica, Ganoderma lucidum, and Pleurotus djamor, respectively. High amounts of carbohydrates, protein, fiber, and ash were recorded for all the species, with Ganoderma lucidum having the highest fiber content of 34.85 ± 0.74 %. Mg, Ca, and Fe were significantly higher in Ganoderma lucidum, while K is at the highest level in Calocybe indica (30119.05 mg/kg). These findings suggest that these mushrooms are potent sources of vital nutrients, with Ganoderma lucidum having superior antioxidant relevance. This research provides an indispensable basis for mushroom choice, formulation of functional foods and nutraceuticals, and optimization of health-promoting characteristics of the studied mushrooms.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Bethel Onyeka Ekute, Muluh Emmanuel Khan, Aloysius Akaangee Pam, Jude Ehwevwerhere Emurotu (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- M. A. Lala, O. A. Adesina, O. J. Odejobi, J. A. Sonibare, Theoretical Air Requirement and Combustion Flue Gases Analysis for Indigenous Biomass Combustion , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 4, November 2022
- Olugbenga Oludayo Oluwasina, Analysis of Adenanthera pavonine L. (Febaceae) Pod and Seed as Potential Pyrolysis Feedstock for Energy production , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 2, May 2022
- N. D. Umar, O. V. Omonona, C. O. Okogbue, Groundwater Quality Assessment Using Multivariate Analysis and Water Quality Index in some Saline Fields of Central Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- S. Iyakwari, H. J. Glass, G. K. Rollinson, A. A. Umbugadu, O. D. Opaluwa, B. O. Frankie, Validation of Near InfraRed preconcentration strategies for ore sorting , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 1, February 2022
- A. Y. Jimoh, M. B. Saadu, A. A. Adetoro, J. Ajadi, T. Issa, U. Issa, Sedimentological and geochemical evaluation of sandstones of the Ilaro formation, Dahomey Basin, Southwestern Nigeria : Insights into paleoenvironments, provenance, and tectonic settings , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 1, February 2024
- Solomon Nehemiah Yusuf, Williams Midala Wakili, Geological mapping and structural analysis from satellite imagery of a section of the Adamawa Massif: Implications for mineralization , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 3, August 2025
- Yusuf Olanrewaju Saheed, Mufutau Abiodun Salawu, Aderemi Babatunde Alabi, Mechanical Evaluation and Minerals Phases Identification of Fine and Coarse Okelele Block Clay Composites for Furnace Lining Application , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 1, February 2022
- V Umarani, A Julian, J Deepa, Sentiment Analysis using various Machine Learning and Deep Learning Techniques , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- Kazeem A. Tijani, Chinwendu E. Madubueze, Reuben I. Gweryina, Typhoid fever dynamical model with cost-effective optimalcontrol , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 4, November 2023
- Rauf I. Rauf, Ayinde Kayode, Bello A. Hamidu, Bodunwa O. Kikelomo, Alabi O. Olusegun, Enhanced methods for multicollinearity mitigation in stochastic frontier analysis estimation , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 4, November 2024
You may also start an advanced similarity search for this article.






