Safranin O dye removal using Senna fistula activated biomass: Kinetic, equilibrium and thermodynamic studies
Keywords:
Senna fistula, Safranin O, adsorbent, kinetics, thermodynamicsAbstract
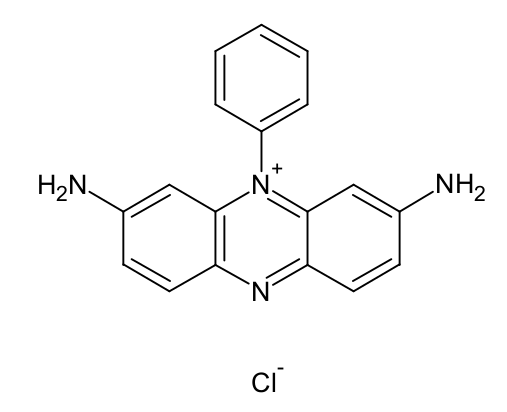
The availability of potable water has decreased in recent times due to the extensive discharge of effluents from some industries. This contaminated water poses a great danger to both human and aquatic life. Senna fistula was activated using phosphoric acid, H3PO4 and its ability to remove Safranin O from aqueous solution was investigated. The characterization of Senna fistula activated carbon was done by Scanning Electron Microscopy and Fourier Transform Infrared Spectroscopy. The impacts of pH, initial dye concentration, contact time, and effect of temperature were investigated. Results showed that the optimum pH for the removal of Safranin O was 4.4. The adsorption capacity increased as the initial dye concentration increased from 30 - 130 mg/L. The dye adsorption equilibrium data were properly fitted to both Freundlich and Langmuir isotherms. The maximum uptake capacity for Safranin O was 22.1 mg/g. The kinetic studies indicated rapid sorption dynamics via a second-order kinetic model. The thermodynamic parameter shows that the sorption of Safranin O on Senna fistula activated carbon was feasible, spontaneous and endothermic. Senna fistula-activated carbon was found to be cheap and efficient adsorbents for the removal of Safranin O dye from aqueous solutions.
Published
How to Cite
Issue
Section
Copyright (c) 2022 C. J. Ajaelu, A. A. Ikotun, E. O. Faboro

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- S. A. Adesokan, A. A. Giwa, I. A. Bello, Removal of Trimethoprim from Water using Carbonized Wood Waste as Adsorbents , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- K. O. Sodeinde, S. O. Olusanya, D. U. Momodu, V. F. Enogheghase, O. S. Lawal, Waste glass: An excellent adsorbent for crystal violet dye, Pb2+ and Cd2+ heavy metals ions decontamination from wastewater , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- Abiodun Oluwatosin Adeoye, Rukayat Oluwatobiloba Quadri, Olayide Samuel Lawal, Sustainable remediation of vancomycin polluted water using pyrolysis biochar of pressed oil palm fruit fibre , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 2, May 2025
- Ibrahim Adamu Mohammed, Majid Khan Majahar Ali, Sani Rabiu, Raja Aqib Shamim, Shahida Shahnawaz, Development and validation of hybrid drying kinetics models with finite element method integration for black paper in a v-groove solar dryer , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 4, November 2025
- F. O. Akinpelu, R. A. Oderinu, A. D. Ohaegbue, Analysis of Hydromagnetic Double Exothermic Chemical Reactive Flow with Convective Cooling through a Porous Medium under Bimolecular Kinetics , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 1, February 2022
- Olugbenga Oludayo Oluwasina, Analysis of Adenanthera pavonine L. (Febaceae) Pod and Seed as Potential Pyrolysis Feedstock for Energy production , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 2, May 2022
- Samsudeen Olanrewaju Azeez, Akeem Adebayo Jimoh, Ismaila Olalekan Saheed, Kabir Opeyemi Otun, Aliru Olajide Mustapha, Folahan Amoo Adekola, Optimization by Box Behnken Design for Eosin Yellow Dye Removal from Aqueous Medium using Date Palm Seeds-Porous Carbon@TiO2 Blend , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 2, May 2022
- Abdullahi Moyosore, Haslina Ahmad, Muhammad Alif Muhammad Latif, Mostafa Yousefzadeh Borzehandani, Mohd Basyaruddin AbdulRahman, Emilia Abdelmalek, Carbon (IV) oxide adsorption efficiency of functionalized HKUST-1, IRMF-1, and UiO-66 metal organic frameworks , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 1, February 2024
- Suha Ibrahim Salih Al-Ali, Zaidun Naji Abudi, Mohammed Nsaif Abbas, Modelling and Simulation for the use of Natural Waste to Purified Contaminated Heavy Metals , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 1, February 2023
- Kehinde Sanni, Adeshola Dauda Adediran, Aliu Olaniyi Tajudeen, Numerical investigation of nonlinear radiative flux of non-Newtonian MHD fluid induced by nonlinear driven multi-physical curved mechanism with variable magnetic field , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 3, August 2023
You may also start an advanced similarity search for this article.






