A model for the control of transmission dynamics of human monkeypox disease in Sub-Saharan Africa
Keywords:
Human monkeypox, Vaccination, Equilibria, StabilityAbstract
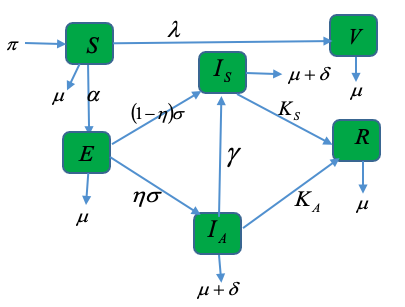
The Human Monkeypox virus has received significant research interest in recent times. While few researchers have studied the effects of vaccination on human-to-animal or animal-to-human Monkeypox transmission, others just studied the effects of treatment on human Monkeypox. In this article, we made the proposition of a deterministic vaccine model that deals with the dynamics of the effects of vaccination and treatment on human Monkeypox in sub-Saharan Africa. We investigated the effects of vaccination on the various epidemiological classes qualitatively. The findings from the analysis of the model are that the model possesses two equilibria, locally asymptotically stable disease-free equilibrium (DFE) when an epidemiological threshold - the effective reproductive number is less than unity, and locally asymptotically stable endemic equilibrium when the number is greater than unity. We then corroborated the theoretical findings with numerical simulations, which reveal that when the rate of vaccination is increased resulting in many newly born persons in the populace being vaccinated, the prevalence of the deadly scourge is significantly reduced, while newly born individuals that miss vaccination experience a drastic torment of the deadly disease that often occasion death. Further revelation from the simulations is that when greater efforts is geared towards vaccination of individuals in the population, the loss of more people to the scourge of the virus would be greatly reduced.
Published
How to Cite
Issue
Section
Copyright (c) 2024 Bolarinwa Bolaji, Abdullahi Ibrahim, Favour Ani, Benjamin Omede, Godwin Acheneje

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- Emmanuel Chidiebere Duru, Godwin Christopher Ezike Mbah, Michael Chimezie Anyanwu, Nnamani Nicholas Topman, Modelling the co-infection of malaria and zika virus disease , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 2, May 2024
- E. A. Nwaibeh, M. K. M. Ali, M. O. Adewole, The dynamics of hybrid-immune and immunodeficient susceptible individuals and the three stages of COVID-19 vaccination , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 3, August 2024
- J. Andrawus, J. Y. Musa, S. Babuba, A. Yusuf, S. Qureshi, U. T. Mustapha, A. Oghenefejiro, I. S. Mamba, Modeling the dynamics of pertussis to assess the influence of timely awareness with optimal control analysis , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 4, November 2025
- Josephine E. Ochigbo, Joel N. Ndam, Wipuni U. Sirisena, Optimal control with the effects of ivermectin and live stock availability on malaria transmission , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 3, August 2024
- Danat Nanle Tanko, Farah Aini Abdullah, Majid K. M Ali, Matthew O. Adewole, James Andrawus, Onchocerciasis control via Caputo-Fabrizio fractional dynamics: a focus on early treatment and vector management strategies , Journal of the Nigerian Society of Physical Sciences: Volume 8, Issue 1, February 2026 (In Progress)
- L. Adamu, N. Hussaini, An Epidemic Model of Zoonotic Visceral Leishmaniasis with Time Delay , Journal of the Nigerian Society of Physical Sciences: Volume 1, Issue 1, February 2019
- James Andrawus, Kayode Isaac Omotoso, Agada Apeh Andrew, Felix Yakubu Eguda, Sunday Babuba, Kabiru Garba Ibrahim, Mathematical model analysis on the significance of surveillance and awareness on the transmission dynamics of diphtheria , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 4, November 2025
- Peter Urane Achimugwu, Mathew Ngugi Kinyanjui, David Mumo Malonza, Analysis of a fractional order climate model due to excessive emission and accumulation of carbon dioxide in the atmosphere , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 4, November 2023
- Elsammani Ali Shokralla, Improving the thermal stability and dielectric properties of epoxy/phenolic resin type (novolac) composites by incorporating carbon nanofibers (CNFs) , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 1, February 2025
- Abiola T. Owolabi, Kayode Ayinde, Taiwo J. Adejumo, Wakeel A. Kasali, Emmanuel T. Adewuyi, Comparative Analysis of the Implication of Periods Before and During Vaccination of COVID-19 Infection in Some Regional Leading African Countries , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 2, May 2022
You may also start an advanced similarity search for this article.
Most read articles by the same author(s)
- Bolarinwa Bolaji, B. I. Omede, U. B. Odionyenma, P. B. Ojih, Abdullahi A. Ibrahim, Modelling the transmission dynamics of Omicron variant of COVID-19 in densely populated city of Lagos in Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 2, May 2023






