Comparative analysis of lithium enrichment mechanisms in aquifers in the Benue Trough
Keywords:
Lithium concentrations, Ground water, Benue TroughAbstract
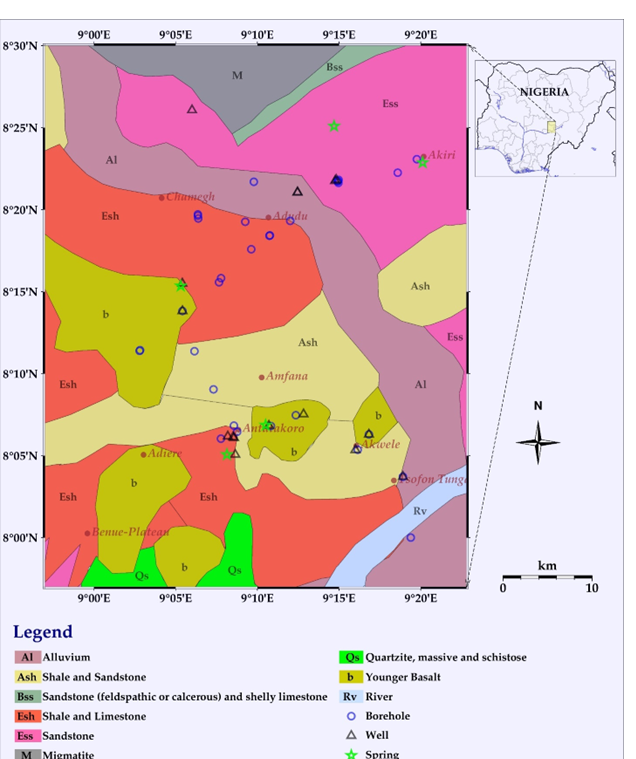
This study investigates the extractability of lithium for energy use from groundwater sources in the Awe part of the Middle Benue Trough. Field measurements of electrical conductivity (EC), total dissolved solids (TDS), temperature, and pH were conducted using a portable meter. Lithium concentrations in 53 groundwater sources, including 17 well samples, 31 borehole samples, and 5 springs were sampled and analysed using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Oxygen isotopes (?18O) were analysed using CO2 equilibration, and hydrogen isotopes (?2H) were analysed by thermochemical reduction to characterize the aquifers. The results indicate that three well water samples (A18, A19, and A38a) have lithium concentrations within seawater values (100-200 µg/L). Similarly, three borehole samples (A6, A15, and A38b) fall within this range, while three other borehole samples A17(794.6 µg/L), A35a(1,826 µg/L), and A37b(330.2 µg/L) exhibit significantly higher concentrations respectively. Among the spring water samples, three samples have lithium concentrations below seawater values, while the remaining two samples A13(1,810 µg/L) and A35b(1,968 µg/L) show elevated levels. Isotopic analysis of ?2H and ?18O identified three distinct types of aquifers in the study area. Water from the deeper aquifer contains high concentrations of lithium, TDS, EC, and elevated temperatures. The lithium concentrations in the deeper saline aquifers A13(1810 µg/L) and A35b(1968 µg/L)suggest significant potential for extraction and use as an energy source.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Rhoda Bernard Gusikit, Solomon Nehemiah Yusuf, Hyeladi Usman Dibal, Victor Bulus Diyelmak, Ahmed Isah Haruna

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Similar Articles
- N. D. Umar, O. V. Omonona, C. O. Okogbue, Groundwater Quality Assessment Using Multivariate Analysis and Water Quality Index in some Saline Fields of Central Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
- C. Gopi, Edward Anand E, A. Charles, C. Manivannan, S .Ponsadai Lakshmi, A. Jose, M. Muthiyan, Physico-Chemical and Trace Metal Analysis in Groundwater of Nagapattinam Region in Nagapattinam District of Tamilnadu State , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 2, May 2023
- Matthew Tikpangi Kolo, Oyeleke Olarinoye, Simon Olonkwoh Salihu, Hyginus Anayo Ugwuanyi, Paul Onuche, Opeyemi Falade, Nwachukwu Chibueze, Annual Effective Dose and Excess Lifetime Cancer Risk due to Ingestion and Inhalation of Radon in Groundwater of Bosso Community Minna, North-Central Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 2, May 2023
- O. T. Fatunsin, O. F. Adeyeye, K. O. Olayinka, T. O. Oluseyi, Effect of pH on the Leaching of Potentially Toxic Metals from Different Types of Used Cooking Pots , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 4, November 2022
- N. K. Olasunkanmi, D. T. Ogundele, V. T. Olayemi, W. A. Yahya, A. R. Olasunkanmi, Z. O. Yusuf, S. A. Aderoju, Assessing leachate contamination and groundwater vulnerability in urban dumpsites: a case study of the Ipata Area, Ilorin, Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 6, Issue 2, May 2024
- Emmanuel P. Agbo, Golden C. Offorson, Abubakar S. Yusuf, John O. Bassey, Moses A. Okono, Ugochukwu Nkajoe, Patrick O. Ushie, Innovative trend analysis of precipitation changes over Nigeria: A case study of locations across the Niger and Benue Rivers , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 1, February 2025
- S. E. Shaibu, E. J. Inam, E. A. Moses, U. A. Ofon, O. K. Fatunla, C. O. Obadimu, N. D. Ibuotenang, N. O. Offiong, V. F. Ekpo, T. J. Adeoye, E. L. Udokang, D. P. Fapojuwo, Prospects of nanosorption and photocatalysis in remediation of oil spills , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 1, February 2023
- K. M. Omatolaa, A. D. Onojah, A. N. Amah, I. Ahemen, Synthesis and characterization of silica xerogel and aerogel from rice husk ash and pulverized beach sand via sol-gel route , Journal of the Nigerian Society of Physical Sciences: Volume 5, Issue 4, November 2023
- E. P. Onokare, L. O. Odokuma, F. D. Sikoki, B. M. Nziwu, P. O. Iniaghe, J. C. Ossai, Physicochemical Characteristics and Toxicity Studies of Crude Oil, Dispersant and Crude Oil-Dispersant Test Media to Marine Organisms , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 1, February 2022
- D. D. Bwede, R. A. Wuana, G. O. Egah, A. U. Itodo, E. Ogah, E. A. Yerima, A. I. Ibrahim, Characterization and Evaluation of Human Health Risk of Heavy Metals in Tin Mine Tailings in Selected Area of Plateau State, Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 3, Issue 4, November 2021
You may also start an advanced similarity search for this article.
Most read articles by the same author(s)
- Solomon Nehemiah Yusuf, Williams Midala Wakili, Geological mapping and structural analysis from satellite imagery of a section of the Adamawa Massif: Implications for mineralization , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 3, August 2025






