Removal of Trimethoprim from Water using Carbonized Wood Waste as Adsorbents
Keywords:
Phosphoric acid (H3PO4), Zinc chloride (ZnCl2), Daniellia-oliveri sawdust, Activated carbon, TrimethoprimAbstract
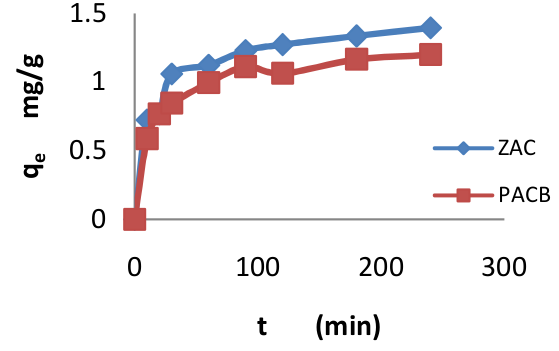
Daniellia—oliveri sawdust-based adsorbents were employed to remove trimethoprim (TMP) from water. The sawdust was thermally carbonized and activated in-stu with ZnCl2 and H3PO4 separately. The adsorbents surface features were profiled using scanning electron microscopic (SEM) and pH point of zero charge (pHpzc ) analyses. The prospects of the adsorbents for the removal of trimethoprim from water were verified. The adsorption processes were performed under different experimental conditions. The adsorption isotherm, the kinetics, and the thermodynamics were studied; and the data fitting output revealed that both chemisorptions and physisorption occurred. Surface and pore diffusion played active role in the adsorption of TMP by the adsorbents. The optimum conditions for adsorption of TMP by the adsorbents were pH at slightly acidic to neutral medium and temperature at room temperature. The fitting isotherm models were: Langmuir (R2 = 0.993) for the zinc-chloride-activated-carbon, Temkin (R2 = 0.962) for the phosphoric-acid-activated-carbon, and the kinetics: pseudo-second order (R2 = 0.997) for both. The maximum monolayer adsorption capacities of the adsorbents for TMP was 4.115 and 6.495 mg/g, respectively. The thermodynamic parameters determined suggested feasibility, spontaneity, and endothermicity of the adsorption processes. The results reveal that the adsorbents were good
prospects for the removal of TMP from water.
Published
How to Cite
Issue
Section
Copyright (c) 2021 Journal of the Nigerian Society of Physical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Most read articles by the same author(s)
- S. A. Adesokan, A. A. Giwa, I. A. Bello, Environmental, Health and Economic Implications of Emerging Contaminants in Nigeria Environment , Journal of the Nigerian Society of Physical Sciences: Volume 4, Issue 3, August 2022
- N. Kure, H. I. Daniel, C. G. Afuwai, E. J. Adoyi, I. A. Bello, The Delineation of Groundwater and Geotechnical Parameters within Marmara Area of Chikun Local Government of Kaduna State, Nigeria , Journal of the Nigerian Society of Physical Sciences: Volume 1, Issue 1, February 2019






