Onchocerciasis control via Caputo-Fabrizio fractional dynamics: a focus on early treatment and vector management strategies
Keywords:
Caputo-Fabrizio fractional derivative, Existence, Stability, Fractional Adams-Bashforth Numerical scheme, Onchocerciasis modelAbstract
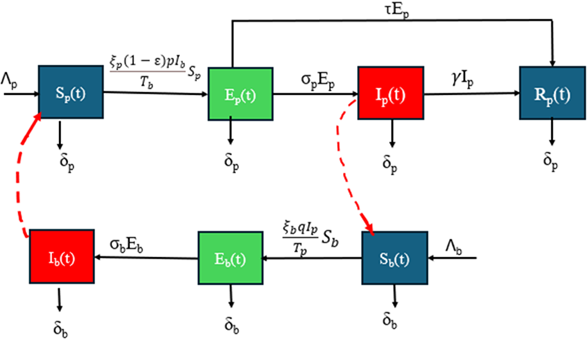
The socio-economic burdens of onchocerciasis have prompted the formulation of several mathematical models to better comprehend the epidemic. However, existing models either use integer-order derivatives, which often do not capture the memory and non-local effects seen in infectious diseases, or fractional order with singularity kernels, which may inadequately represent memory effects due to their singularity kernels. Onchocerciasis has a prolonged incubation and slow progression, making past conditions impactful on the disease's current and future course. Fractional derivatives effectively capture this memory effect, providing a more realistic depiction of the infection dynamics than integer-order models. We propose a non-local, non-singular exponential kernel fractional-order onchocerciasis model in the Caputo-Fabrizio fractional derivative sense to capture the disease's memory effects. Our model incorporates early treatment of exposed individuals as a critical intervention parameter, and vector management strategies are also incorporated. Using fixed-point theorem and iterative methods, we establish the existence and uniqueness of solutions, derive conditions for onchocerciasis-free and endemic equilibrium points, and analyze their stability, confirming the model's biological feasibility. Numerical simulations are conducted using a three-step fractional Adams-Bashforth method. Sensitivity analyses indicate that vector management and early treatment effectively reduce the effective reproduction number, while increases in the human-to-vector contact rate elevate it. Numerical results demonstrate that early treatment and vector management can significantly control onchocerciasis. The fractional-order "memory effect" highlights the importance of continuous monitoring and consistent application of control measures to reduce the memory index and curb onchocerciasis prevalence over time.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Danat Nanle Tanko, Farah Aini Abdullah, Majid K. M Ali, Matthew O. Adewole, James Andrawus (Author)

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Most read articles by the same author(s)
- James Andrawus, Kayode Isaac Omotoso, Agada Apeh Andrew, Felix Yakubu Eguda, Sunday Babuba, Kabiru Garba Ibrahim, Mathematical model analysis on the significance of surveillance and awareness on the transmission dynamics of diphtheria , Journal of the Nigerian Society of Physical Sciences: Volume 7, Issue 4, November 2025






