Design, synthesis and studies on thermodynamic and biological activities of lanthanide III complexes of hydrazinecarbothioamide ligands
Keywords:
Tetradentate ligands, Lanthanide complexes, biological activity, Molecular docking, Thermodynamic stabilityAbstract
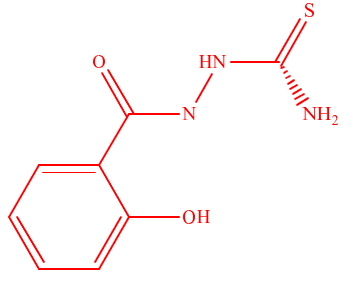
New tetradentate ligands, 2-(2-hydroxybenzoyl)hydrazinecarbothioamide, HL1, and 2-(2-mercaptobenzoyl)hydrazinecarbothioamide, HL2, were designed and synthesized from the condensation reactions of salicylic and, thiosalicylic acids with thiosemicarbazide in one pot without any coupling agent. The ligands were characterized using physical and spectroscopic methods. They formed complexes with Nd (III) and Dy (III) ions. The FTIR results suggest that the ligands exhibit azo-hydrazo tautomerism. The complexes were thermally stable with NdHL1 having a decomposition activation energy, Ea of 54.7 kJmo${}^{-1}$, $\Delta$H value of 47.8 kJmol${}^{-1}$, $\Delta$S value of -23.1 kJmol${}^{-1}$, $\Delta$G value of 237.8 kJmo${}^{-1}$ being the most stable at 530 C. The dysprosium complexes were crystalline, while the~neodymium complexes were~amorphous and produced no peaks in X-ray diffraction spectra. The complexes have the general formula [Ln (HL)${}_{2}$(H${}_{2}$O)${}_{\ m}$(NO${}_{3}$)${}_{\ n}$] (H${}_{2}$O)${}_{\ m}$(NO${}_{3}$)${}_{\ n}$, where m ranged between 0 and 2, while n ranged from 0 to 1. The complexes showed thermal stability beyond 400~ $\circ$C. All the compounds showed significant antimicrobial activities against aspergillus flavus at concentrations of 12.5, 25, and, 50 mg/ml, with the highest activity recorded at 50 mg/ml for DyHL2. DyHL2 complex also showed the highest binding affinity of 5.3 kcal/mol, compared to the binding affinities -1.3, kcal/mol, -1.2, -5.2, 5.1, and -5.0 kcal/mol obtained for HL1, HL2, NdHL1, NdHL1, DyHL1 and NdHL2 respectively from molecular docking studies, indicating inhibitory efficacy against A. \textit{f}lavus. The complexes are potential novel compounds for the development of drugs, photocatalysts, photosensitized materials, and photocells used in organic synthesis, and solar energy conversion devices.
Published
How to Cite
Issue
Section
Copyright (c) 2025 U. B. Amadi, M. Ogwuegbu, C. K. Enenebeaku, G. onyedika

This work is licensed under a Creative Commons Attribution 4.0 International License.






